16 Predict Which of the Following Has an Ionic Bond
View the full answer. 2 See answers Advertisement Advertisement OlaMacgregor OlaMacgregor Explanation.
D all of the above.
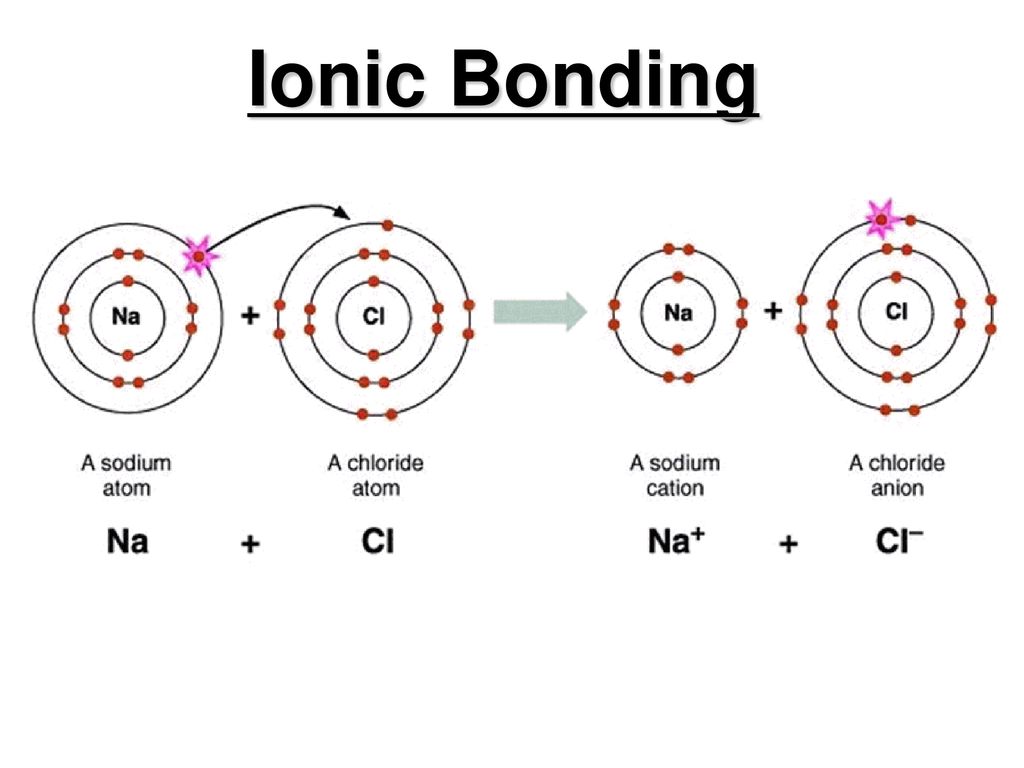
. If the difference is. D all of the above. A covalent bond consists of the mutual sharing of one or more pairs of electrons between two atoms.
A AlCl3 B Cu2s C FeF3 D all of the above E none of the above. CaO CO2 all of the above SO3. Example Exercise 123 Characteristics of Ionic Bonds All of these statements are true regarding an ionic bond.
These electrons are simultaneously attracted by the two atomic nuclei. Get the answer to your homework problem. A bond in which the electronegativity difference between the atoms is between 05 and 20 is called a polar covalent bond.
Which of the following happens when an ionic bond is formed. A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is unequal. In a polar covalent bond sometimes simply called a polar bond the distribution of electrons around the molecule.
A CoO B NO C ZnO D all of the above E none of the above. Predict which of the following has an ionic bond. A HI B IBr C LiH D all of the above E none of the above.
Answer choices shares shares loses gains gains loses loses loses. Predict which of the following has an ionic bond. Predict which of the following has an ionic bond.
Covalent bonds occur between identical atoms or between different atoms whose difference in electronegativity is insufficient to allow transfer of electrons to form ions. See the answer See the answer done loading. An ionic bond results when the sharing is so unequal that fully charged ions form.
Predict whether each of the following ionic compounds is soluble in water. One atom becomes more electronegative than another atom. Answer choices 1 electron 8 electrons 16 electrons 32 electrons Question 6 30 seconds Q.
Predict which of the following molecules will have a stronger ionic bond. LiH is formed by the complete transfer of electrons from lithium metal to hydrogen non-metal. C An ionic bond is from the attraction of positive and negative ions.
Mg and O bond by sharing electrons. Think about charges and bond lengths a MgBr2 or MgF2 b Na2O or Rb2O c Ca3PO42 or Li3PO4 Determine the number of valence electrons in each molecule below. The bond formed by the transfer of electrons between a metal and a non-metal is called an ionic bond.
Where q_q_- is product of charges of the ions and r_r_- is the sum of. A An iron atom loses electrons and a sulfur atom gains electrons. Bonding between atoms produces an outermost energy level for each atom filled with.
Two atoms attain equal electronegativities. D TUNICUL LICO 30 30 Predict which of the following has an ionic bond. Get the answer to your homework problem.
Which type of bond is formed between two chlorine atoms in a chlorine molecule. One method to classify bonds based on this difference can be described as follows. The ionic radius of an oxide is greater than its atomic radius.
B The radius of an iron atom is greater than its ionic radius. 19 ______ A SnO2 B CO2 C SO3 D all of the above E none of the above. B More questions like this The atomic number of sulfur is 16.
Predict which of the following has a covalent bond. See the answer Predict which of the following has an ionic bond. 4 Predict which of the following has an ionic bond.
Answer 1 of 2. So the bond formed by lithium and hydrogen in LiH is an ionic bond. If the electronegativities differ by less than 04 units the bond can be classified as nonpolar covalent.
A AlCl3 B Cu2s C FeF3 D all of the above E none of the above. E none of the above. Mg atom gains electrons and oxygen atoms lose electrons.
Try Numerade Free for 7 Days. 1 H2Se 2H2S 3 H2O 4 all of the above 5 none of the above Expert Answer 5 none of the above An ionic bond is the extreme case of a polar covalent bond. E none of the above.
Predict which of the following has an ionic bond. Chemistry questions and answers. Lattice energy LE is proxied by the following formula LE fracq_q_-r_r_-.
1 polar covalent 2 nonpolar covalent 3. This makes option C the correct choice. In an ionic bond the bonding atom.
Predict which of the following compounds are ionic and which are covalent based on the location of their constituent atoms in the periodic table. D TUNICUL LICO 30 30 Predict which of the following has an ionic bond. Sodium forms an ionic bond with chlorine when sodium ____ an electron and chlorine ____ an electron.
University of Manchester. Predict whether each of the following bonds is ionic polar covalent or nonpolar covalent. The ionic radius of an oxide is greater than its atomic radius.
A HI B IBr C LiH D all of the above E none of the above. D all of the above. Answered Sep 12 2016 by Kygok.
AgCl all of thc abovc BrCI HCI. Predict which of the following has an ionic bond. 5 Predict which of the following has an ionic bond.
We commonly use lattice energy to evaluate the strength of an ionic bond. One atom pulls an electron from another atom. Solved Predict which of the following has an ionic bond.
Forming an ionic bond between Mg2 and O2- requires energy. E none of the above. OOOOOOOO Electronegativity difference can be used to predict bond type.
Chemistry questions and answers. Try Numerade Free for 7 Days. A C4H10O b CH3NO2 c POCl2-1 d HBrO3 Draw full Lewis.
Predict which of the following compounds are ionic and which are covalent based on the location of their constituent atoms in the periodic table. 6 Predict which of the following has an ionic bond. Sulfur combines with hydrogen by covalent bonding to form a.
Based on electronegativity trends in the periodic table predict which of the following compounds will have the greatest ionic character in its bonds. Predict which of the following has an ionic bond. Asked Sep 12 2016 in Chemistry by Garrett.
It is known that in order to determine a compound to be ionic polar covalent or non-polar covalent it is necessary to determine the.
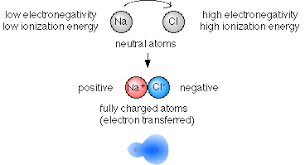
Formation Of Ionic Bond And Factors Affecting It Variable Electrovalency

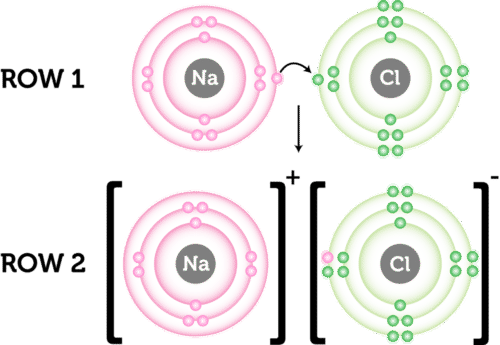
No comments for "16 Predict Which of the Following Has an Ionic Bond"
Post a Comment